Beating Pharma at Their Own Game
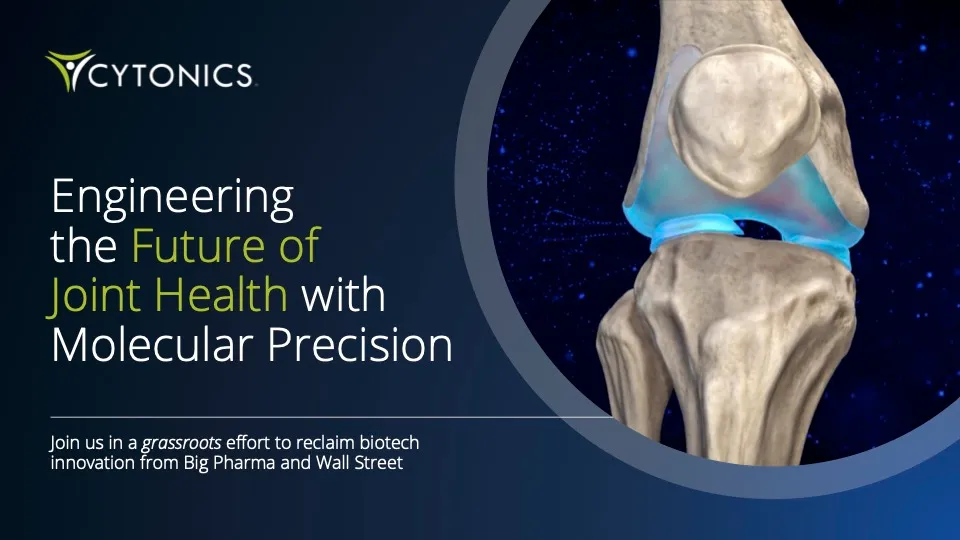
Osteoarthritis (OA) is an epidemic affecting 500M+ people, causing debilitating pain, complete loss of mobility, and with no real cure. Cytonics is righting Big Pharma’s repeated failures by developing the first truly disease-modifying therapy for OA.
10,000+ patients treated with first-generation therapy
$25M+ raised from 7,000+ investors, with $2M from pro athletes
Tracking toward a potential exit by 2028
Join this grassroots effort to reclaim biotech innovation from Big Pharma and Wall Street as an early-stage investor.
10,000+ Patients Treated. $25M+ raised.
26 patents.
10,000+ patients already treated with first-gen therapy


Partnered with leading researchers like Stanford and Scripps
The $25M raised from equity crowdfunding investors
Get the investor deck
Received $1.8M in National Institute of Health grants
CYT-108’s Phase 1 FDA human clinical trial completed; safety confirmed
25+ patents issued worldwide in key global markets (US, EU, CA, JP, AU)
Exclusive Investor Perks
Previous investors receive an additional 10% in bonus shares on top of the perks listed below. Shares will be issued and reconciled by the completion of the raise.
Osteoarthritis Therapeutics is a $560B+ 1 Market
There is no disability more common worldwide than arthritis, with osteoarthritis (OA) making up nearly half of those cases. That’s why its market for therapeutics is worth $560B+. With rates growing 132%2 worldwide between 1990 and 2020, and prevalence expected to rise another 60-100% by 2050, we need answers.

But No Drug Works
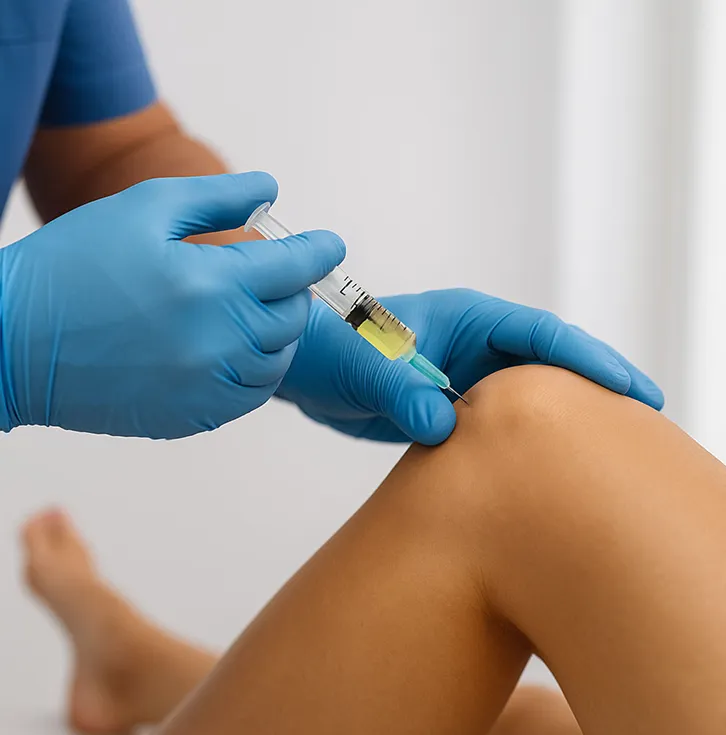
That means 500M+ global patients are getting short-term fixes like anti-inflammatories and corticosteroid shots. These may dull pain, but the disease still progresses toward costly, painful, and invasive joint replacements. We believe that this is unacceptable.
Big Pharma has spent $1B+ chasing a true “disease-modifying” therapy capable of halting OA in its tracks and repairing cartilage tissue, but their strategy has failed to appreciate the multi-faceted nature of the disease and they’ve consistently come up short.

Here’s Why Big Pharma is Failing
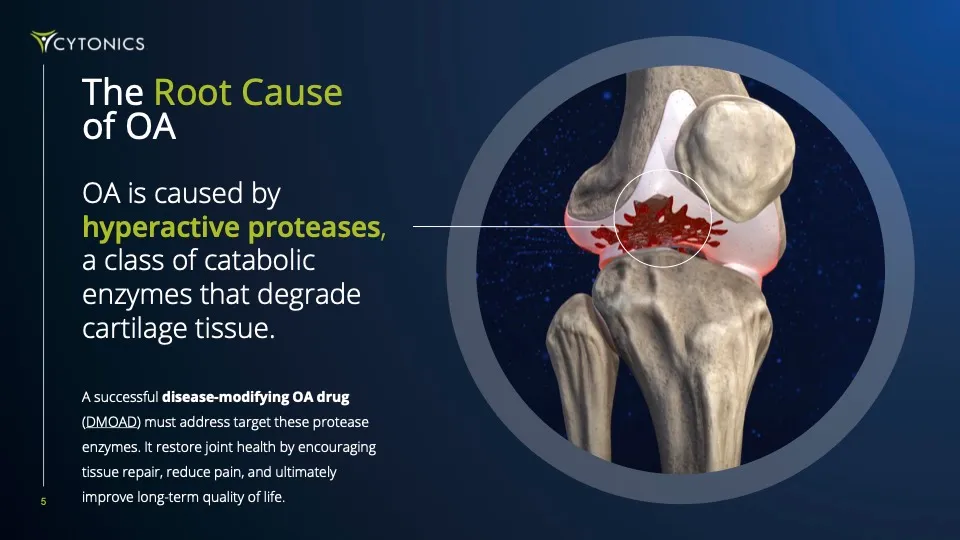
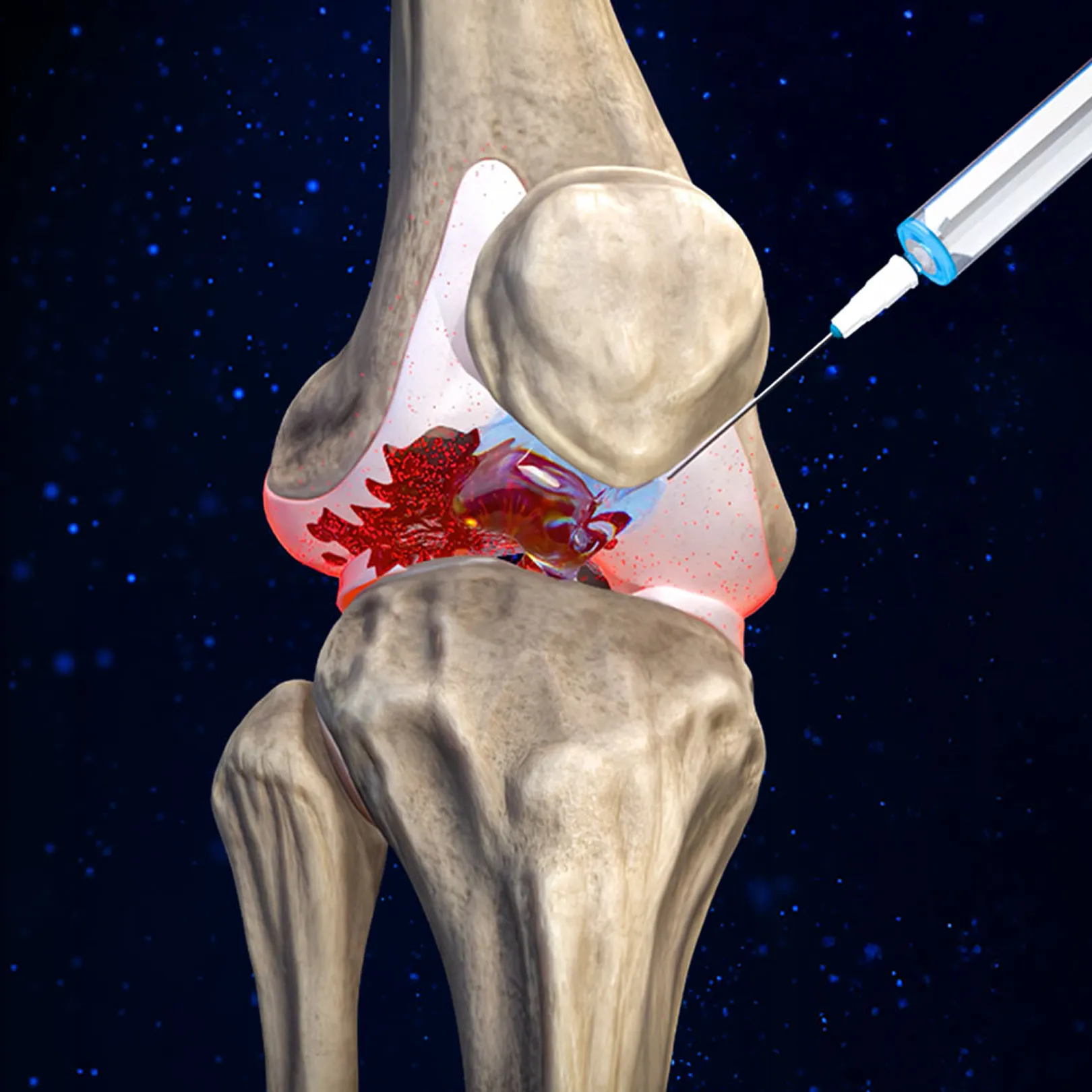
Every drug fails for the same reason: they go after just one target. But OA is complex, caused by a team of cartilage-destroying enzymes called “proteases”. If you don’t stop them all, the damage continues and the disease spirals out of control. Further compounding their shortsighted approach, they’ve attempted to deliver drugs orally, not directly into the joint.
Our Breakthrough: CYT-108
The result: a powerful dual mechanism of disease-modification that may be able to halt and repair joint damage at the molecular level.
How CYT-108 Works
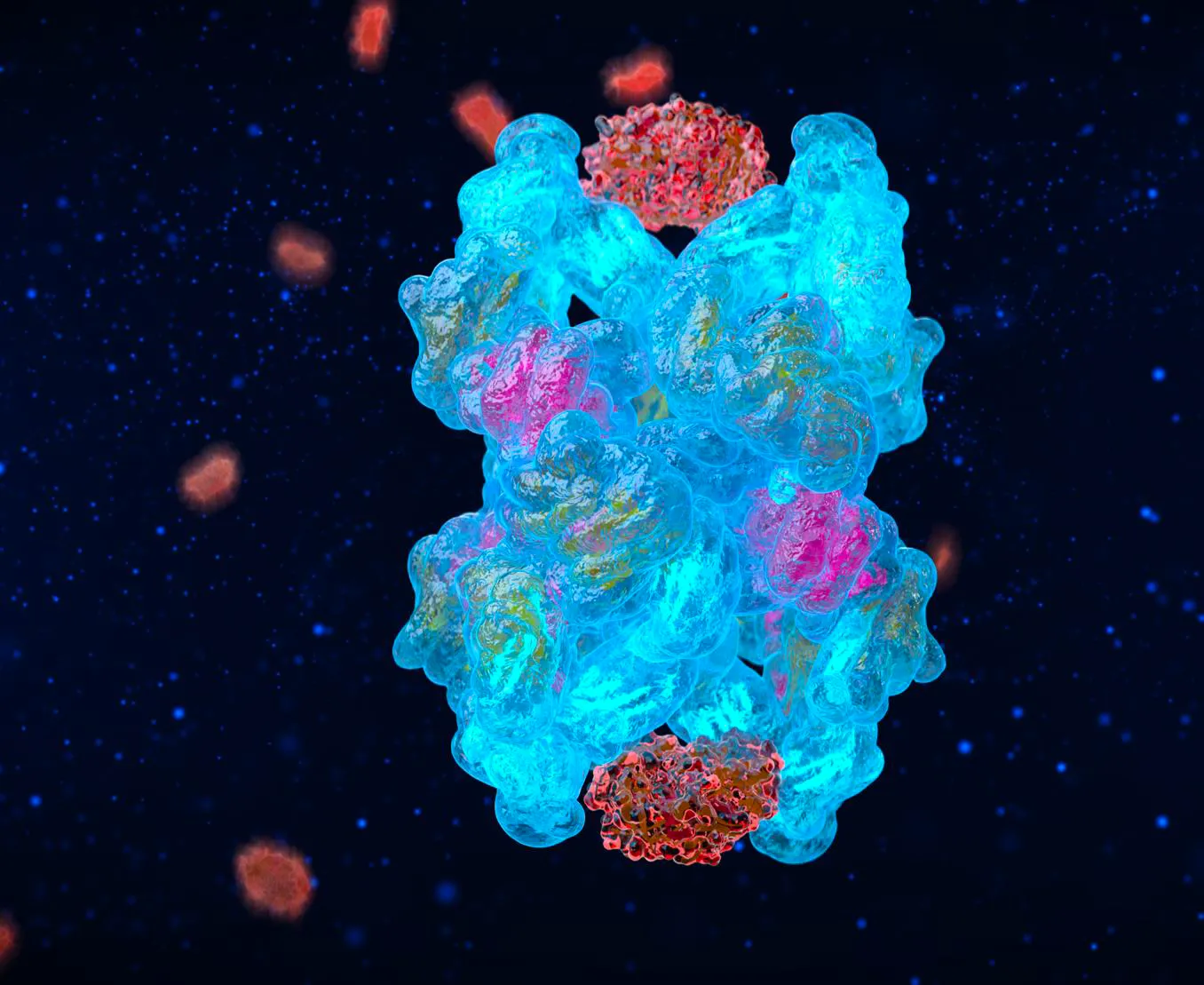
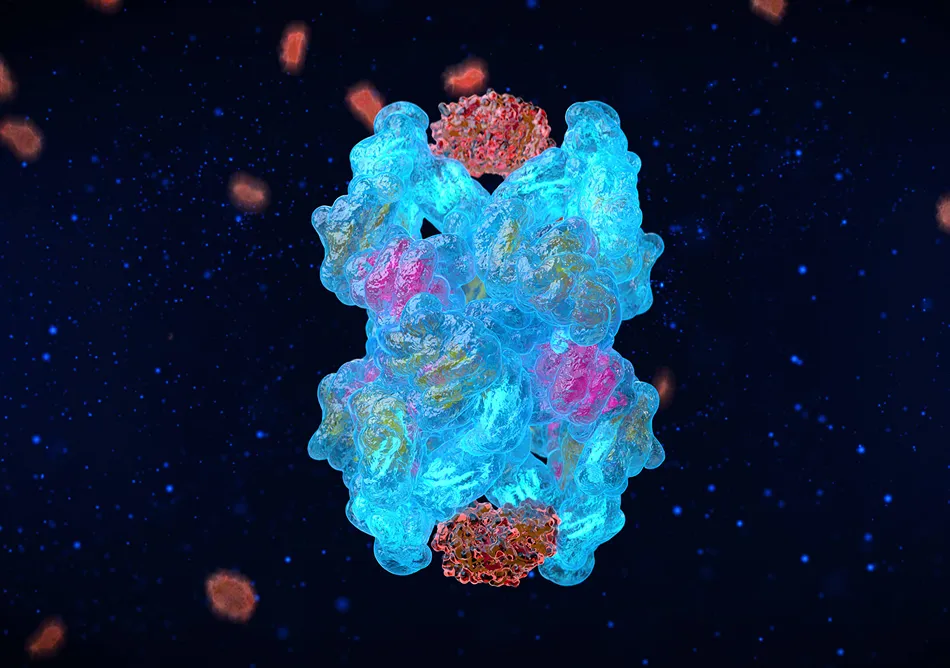
CYT-108 is a genetically engineered “super A2M” protein that is >200% more potent than the naturally-occurring A2M protein
A2M is a naturally-occurring protease inhibitor that protects against cartilage degradation, but levels are too low in joints to be an effective treatment.
CYT-108 is delivered in massive, supraphysiological concentrations (17x natural A2M levels in the joint) for extremely potent inhibition of proteases and stimulation of cartilage-secreting chondrocytes.
1
2
3


A2M: Leveraging Natures Beautiful Design
At the core of our technology is Alpha-2-macroglobulin (A2M) - a naturally occurring protein that acts as the body’s molecular defense system. It is found in the blood stream and plays a role in blood clotting through a mechanism called protease inhibition. Cartilage breakdown in arthritis is also caused by these destructive proteases. Cytonics hypothesized that A2M’s mechanism of action could be leveraged to inhibit the multitude of protease enzymes that drive cartilage breakdown in arthritic joints.
A2M is one of the most well-studied proteins in human biology. Its structure was crystallized in 2012, and more than 90,000 peer-reviewed studies support its role as abroad-spectrum protease inhibitor. Its two “bait regions” function like a molecular Venus Flytrap, binding and deactivating these destructive enzymes. A2M is such a potent protease destroyer that it has been dubbed the Physiological Guardian because of its ability to protect many different tissue types throughout the body.
“So, if the human body is already full of A2M in the bloodstream, then why doesn’t it just naturally protect joints from arthritic decay?”
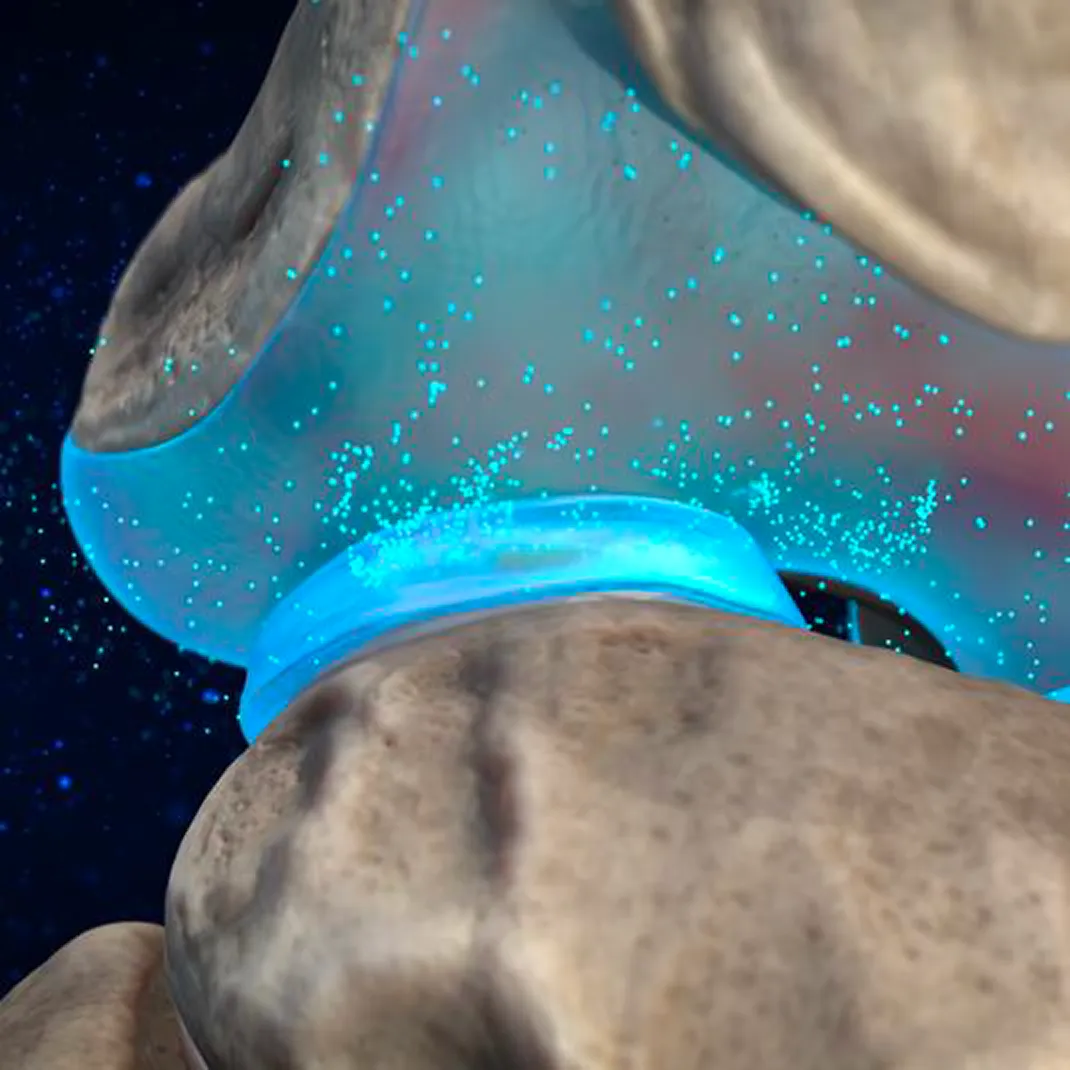
Cytonics’ approach is simple: Leverage the therapeutic power of A2M by delivering high concentrations of this miraculous protein directly into arthritic joints to halt cartilage damage, reduce joint membrane inflammation, and stimulate cartilage regrowth.
APIC™: Harnessing the Power of A2M
The First Generation Technology
10,000+
Patients Treated
300+
Physicians Trained
200%
More Potent (CYT-108)
“Regenerative orthopedics” has been dominated by stem cells, PRP, and other autologous treatments - many unproven, unregulated, and not reimbursed by insurance payers. This is where Cytonics broke away from the pack.
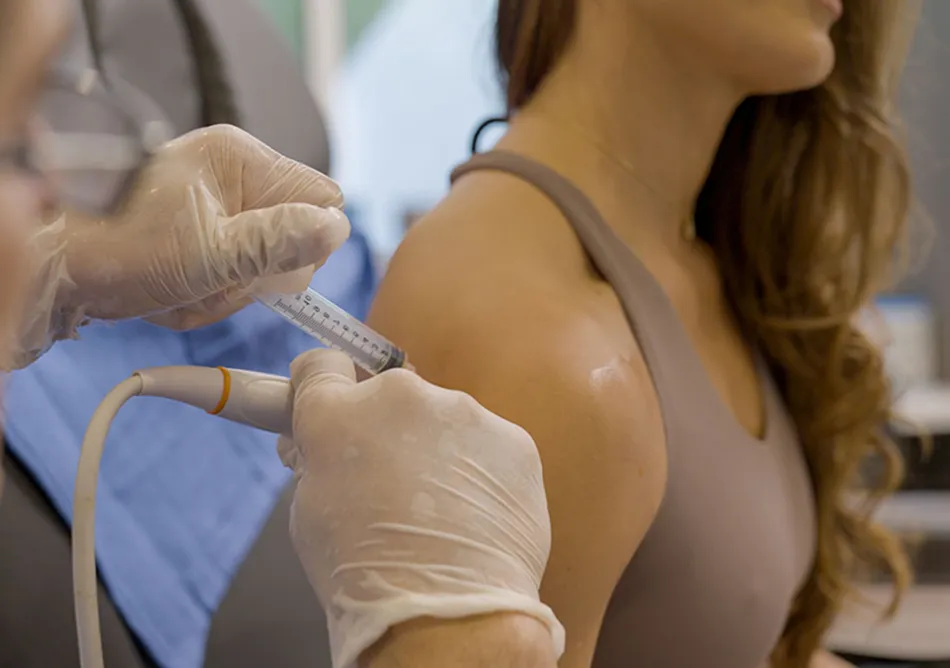
Our first-generation therapy, APIC™ (Autologous Protease Inhibitor Concentrate), put Alpha-2-Macroglobulin (A2M) on the clinical map and into the offices of orthopedists nationwide. Using a proprietary filtration system, APIC™ isolates and concentrates A2M from a patient’s blood and re-injects it into the joint to inhibit cartilage breakdown and promote regeneration at the molecular level.
But as effective as APIC™ is, it has limitations: variable A2M levels between patients, multi-hour processing, and equipment requirements that constrain scalability.
These constraints inspired the next evolution in our platform: an engineered, highly potent version of A2M designed for more potent treatment on a global scale for wide-spread accessibility: We called this miraculous therapy CYT-108.
From APIC™ to CYT-108: The Next Generation of A2M Therapies
CYT-108 was designed to address APIC™’s limitations. Using recombinant genetic engineering, we created a “super A2M” variant that is 200% more potent than the natural A2M and can be produced on a global scale in consistent, supraphysiological concentrations and dosed precisely in every patient and every joint.
This is a true biopharmaceutical approach to solving osteoarthritis on a global scale.
.webp)
Get the investor deck

Strategically Partnered with Rener & Eve Gracie
A third-generation Brazilian Jiu-Jitsu master and former WWE champion, Rener and Eve Gracie’s investment in Cytonics carries real weight. As two of the world’s most respected figures in athletics, injury prevention, and health advocacy, they bring credibility, visibility, and real-world expertise.

Health & Performance Advocacy:
Real-World Alignment:
Brand Expansion & Awareness:
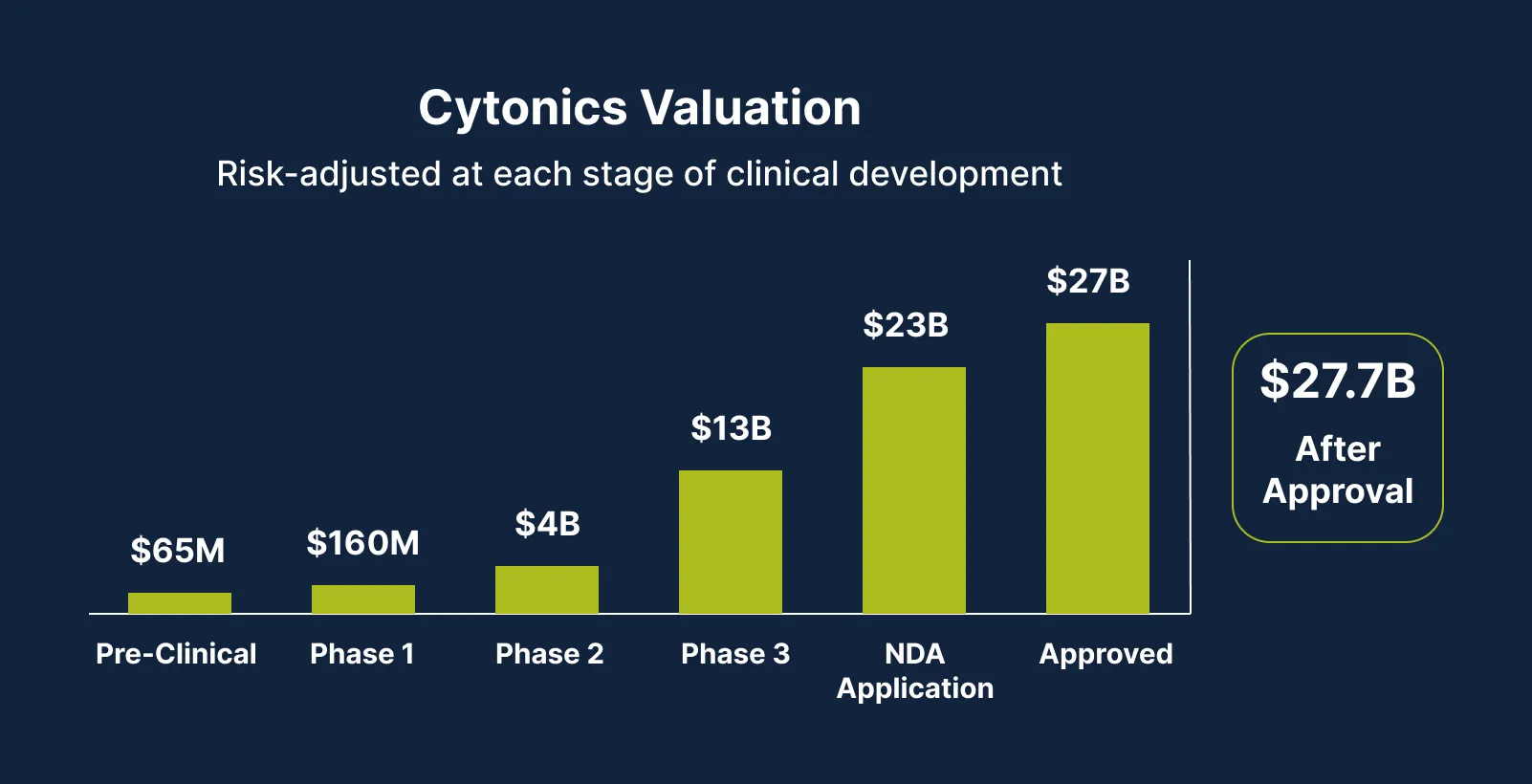
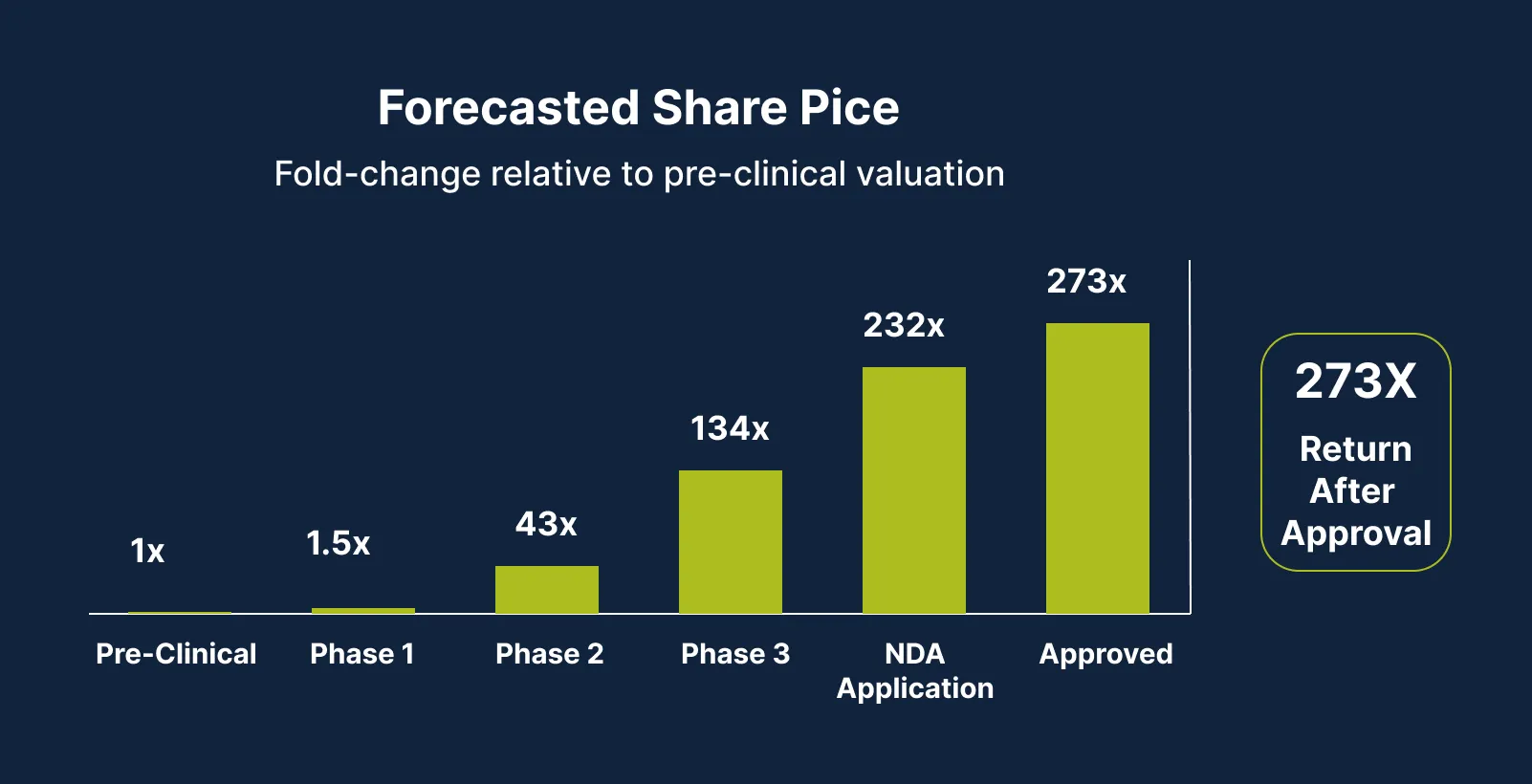
Our Path to Potential Long‑Term Share Price Appreciation
This financing values the company at approximately $160M. Management aims to create meaningful long-term shareholder value. Here is how we plan to do that. 3
2025
- Completed first-in-human Phase 1 clinical trial
- No drug-related adverse events were detected, confirming CYT-108’s safety
- Prepared next clinical trial (Phase 1b/2a) protocol
- Onboarded statistician, regulatory consultant (former FDA reviewer), and medical writers
- Initiated new Oncology program (focus on metastatic melanoma)
2026
- Publish Phase 1 Clinical Study Report (Q1)
- GMP manufacturing run of CYT-108 (Q1)
- Reformulation studies to concentrate CYT-108 (Q2)
- Conduct PK/PD studies to establish dose range for Phase 1b/2a studies (Q2)
2027–2028
- Hold Type C meeting with the FDA to get feedback on Phase 1b/2a trial design (Q1 ‘27)
- File Investigational New Drug Application (Q2 ‘27)
- Launch Phase 1b/2a efficacy study (Q2 ‘27)
- Complete Phase 1b/2a study (Q2 ‘28)
- Hold End-of-Phase 2 meeting with FDA (Q3 ‘28)
2029+
- Initiate Phase 2b trial (Q1 ’29 to Q1 ‘30)
- Position for IPO, acquisition, or major partnership to fund global commercialization
- Prepare regulatory submissions in US, EU, and select global markets
- Continue to expand development pipeline into other disease areas (e.g., oncology)
- Pursue clinical development of additional A2M-based therapies
"These statements reflect management’s current views based on information currently available and are subject to risks and uncertainties that could cause the company’s actual results to differ materially. Investors are cautioned not to place undue reliance on these forward-looking statements as they are meant for illustrative purposes and they do not represent guarantees of future results, levels of activity, performance, or achievements, all of which cannot be made. Moreover, no person nor any other person or entity assumes responsibility for the accuracy and completeness of forward-looking statements, and is under no duty to update any such statements to conform them to actual results. Please see Data Room for additional detail regarding the assumptions underlying these projections."
Get the investor deck

A Grassroots Effort: For the People, By the People
We’re part of a grassroots movement to reclaim biotech innovation from Big Pharma and Wall Street. For decades, early-stage biotech investing was reserved for VCs, hedge funds, and “institutional investors.” By inviting individuals like yourself to invest, we’re shattering the glass ceiling and democratizing both pharmaceutical development investing. Cytonics has flipped the script and is allowing you to share in the growth of breakthrough science that tackles massive unmet needs like osteoarthritis. Big Pharma and Venture Capitalists need not apply.

“As a patient who has personally benefited from the Cytonics A2M therapy, I asked if I could be a partner to help spread the word. I have seen far too many times people get sidelined from their passions because their bodies don't respond and their bodies don't work and they exist in chronic pain. So if we can help reduce or eliminate that pain and delay the onset of chronic osteoarthritis, I'm all for it, sign me up."
Co-founder of Gracie University
Strategic partner/investor in Cytonics
Led by Seasons Scientists, Physicians, and Industry Outsiders
Our leadership combines world-class scientific expertise, decades of clinical experience, and a proven track record in biotech R&D with the first-principles thinking of biotech neophytes (our Founder and CEO). Our Board of Directors and Medical Advisory Board have dominated the last decade of osteoarthritis research (a combined 80 osteoarthritis clinical trials), developed FDA-approved biologics at major pharma companies, and raised millions in combined capital. This unique combination of traditional biotech experience with avant garde thinking of industry outsiders gives us a strategic advantage as we tackle a problem that the Big Pharma giants have failed to solve.

- Proven innovator in orthopedic medicine, with 45+ peer-reviewed publications
- Served as faculty at Stanford and brings 30+ years of surgical experience
- Deep patient insight, leveraging decades of frontline experience treating joint degeneration
- 5th degree black belt jiu jitsu

- 15+ years in biotechnology R&D with healthcare investment banking experience
- Led funding efforts to secure $25M in non-VC investor backing
- Proven track record in clinical trial execution, patent protection, and securing licensing opportunities
- Johns Hopkins trained biomedical engineer with specialization in recombinant protein engineering

- GMP and regulatory affairs veteran with deep experience in biologics and vaccine programs
- Trained microbiologist and immunologist
- Built and led quality systems and global inspection readiness efforts
- Supported INDs reviewed by FDA, EMA, MHRA, and NMPA
- Former BARDA and FDA Office of the Commissioner leader in medical countermeasures
- Leads GMP-compliant manufacturing of CYT-108 at Cytonics from day one

- Responsible for communicating Cytonics’ science, strategy, and long-term vision to investors and the public
- Specializes in translating complex biology into clear, disciplined narratives
- Extensive experience with early- and growth-stage companies in regulated, capital-intensive industries
- Oversees brand development, campaign strategy, and digital advertising supporting capital formation and investor education
- Focuses on precision-driven storytelling that maintains scientific and regulatory integrity

- Architect of FOCAL, the company’s osteoarthritis AI engine
- Applies AI-driven modeling to digitally optimize CYT-108 clinical trials for human success
- Brings 20+ years of experience in AI, cloud computing, data engineering, and security
- Former leader in national-security encryption, offensive security (NTT Com Security), and scaled DigitalOcean from 5,000 to 100,000+ customers
- Dedicated technologist focused on continuous learning across Kubernetes, AI inference, and network security
Frequently Asked Questions
Why invest in startups?
Regulation CF allows investors to invest in startups and early-growth companies. This is different from helping a company raise money on Kickstarter; with Regulation CF Offerings, you aren’t buying products or merchandise - you are buying a piece of a company and helping it grow.
How much can I invest?
Accredited investors can invest as much as they want. But if you are NOT an accredited investor, your investment limit depends on either your annual income or net worth, whichever is greater. If the number is less than $124,000, you can only invest 5% of it. If both are greater than $124,000 then your investment limit is 10%.
How do I calculate my net worth?
To calculate your net worth, just add up all of your assets and subtract all of your liabilities (excluding the value of the person’s primary residence). The resulting sum is your net worth.
What are the tax implications of an equity crowdfunding investment?
We cannot give tax advice, and we encourage you to talk with your accountant or tax advisor before making an investment.
Who can invest in a Regulation CF Offering?
Individuals over 18 years of age can invest.
What do I need to know about early-stage investing? Are these investments risky?
There will always be some risk involved when investing in a startup or small business. And the earlier you get in the more risk that is usually present. If a young company goes out of business, your ownership interest could lose all value. You may have limited voting power to direct the company due to dilution over time. You may also have to wait about five to seven years (if ever) for an exit via acquisition, IPO, etc. Because early-stage companies are still in the process of perfecting their products, services, and business model, nothing is guaranteed. That’s why startups should only be part of a more balanced, overall investment portfolio.
When will I get my investment back?
The Common Stock (the "Shares") of Cytonics (the "Company") are not publicly-traded. As a result, the shares cannot be easily traded or sold. As an investor in a private company, you typically look to receive a return on your investment under the following scenarios: The Company gets acquired by another company. The Company goes public (makes an initial public offering). In those instances, you receive your pro-rata share of the distributions that occur, in the case of acquisition, or you can sell your shares on an exchange. These are both considered long-term exits, taking approximately 5-10 years (and often longer) to see the possibility for an exit. It can sometimes take years to build companies. Sometimes there will not be any return, as a result of business failure.
Can I sell my shares?
Shares sold via Regulation Crowdfunding offerings have a one-year lockup period before those shares can be sold under certain conditions.
Exceptions to limitations on selling shares during the one-year lockup period:
In the event of death, divorce, or similar circumstance, shares can be transferred to:
• The company that issued the securities;
• An accredited investor;
• A family member (child, stepchild, grandchild, parent, stepparent, grandparent, spouse or equivalent, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, or sister-in-law, including adoptive relationships).
What happens if a company does not reach their funding target?
If a company does not reach their minimum funding target, all funds will be returned to the investors after the close of the offering.
How can I learn more about a company's offering?
All available disclosure information can be found on the offering pages for our Regulation Crowdfunding offering.
What if I change my mind about investing?
You can cancel your investment at any time, for any reason, until 48 hours prior to a closing occurring. If you’ve already funded your investment and your funds are in escrow, your funds will be promptly refunded to you upon cancellation. To submit a request to cancel your investment please email: info@dealmakersecurities.com
How do I keep up with how the company is doing?
At a minimum, the company will be filing with the SEC and posting on its website an annual report, along with certified financial statements. Those should be available 120 days after the fiscal year end. If the company meets a reporting exception, or eventually has to file more reported information to the SEC, the reporting described above may end. If these reports end, you may not continually have current financial information about the company.
What relationship does the company have with DealMaker Securities?
Once an offering ends, the company may continue its relationship with DealMaker Securities for additional offerings in the future. DealMaker Securities’ affiliates may also provide ongoing services to the company. There is no guarantee any services will continue after the offering ends.