$25M+
Funding to Date
Funding to Date
$390B Market Opportunity
Over 6,000 Equity Shareholders
Partnerships With Industry Leaders


*The above testimonials may not be representative of the opinions of other medical experts or the experiences of other customers and is not a guarantee of future performance or success.
Receive Exclusive Investor Updates
Oops! Something went wrong while submitting the form.
By clicking "Learn More", you consent to receive marketing text messages (e.g. promos, cart reminders) from StartEngine Crowdfunding, Inc. at the number provided, including messages sent by autodialer. Consent is not a condition of purchase. Msg & data rates may apply. Msg frequency varies. Unsubscribe at any time by replying STOP or clicking the unsubscribe link (where available). Privacy Policy & Terms
Our Goal: Succeed Where Big Pharma Has Failed
Image is a computer-generated demo. CYT-108 is currently under development.
Image is a computer-generated demo. CYT-108 is currently under development.
Osteoarthritis (OA) is a chronic, degenerative joint disease without an effective treatment.
OA treatment has stumped researchers worldwide for more than 30 years, partially because it’s a multi-factorial disease caused by multiple protease enzymes. Big Pharma has attempted to figure out how to target some of the proteases, but not all of them, which is why many current treatments are only able to treat symptoms without addressing the root cause of the disease. Creating a disease-modifying therapy for OA is difficult, so it’s no surprise that Big Pharma hasn't succeeded… but we’re not Big Pharma.
Image is a computer-generated demo. CYT-108 is currently under development.
Cytonics is developing the solution: CYT-108
CYT-108 is a broad-spectrum protease inhibitor that we believe has been honed for anti-protease activity against all classes of proteases involved in OA. Instead of just targeting a portion of the root problem, CYT-108 targets the whole problem. In short, we feel the disease-modifying therapy that Big Pharma hasn’t been able to produce is in Cytonics' hands and under development.
Image is a computer-generated demo. CYT-108 is currently under development.
OA has been a tough problem to solve, largely because of the multi-factorial nature of the disease itself. Big Pharma has shown that they don’t have the answers, but with over 365M people suffering worldwide and a $390B market, Cytonics is stepping in to find an answer.
After careful research and testing, we’ve identified a molecule that could be the key to the solution: Alpha-2-Macroglobulin (A2M)
Image is a computer-generated demo. CYT-108 is currently under development.
Why CYT-108 is a Treatment Worth Believing In:
$25M+ Raised So Far Raised From Over 6K Investors
$25M+ Raised So Far Raised From Over 6K Investors
Osteoarthritis affects over 365 million people worldwide, with no truly effective, long-term treatment available. The global OA market is valued at $390 billion, and even a small market capture presents an enormous opportunity.
By investing now, you are supporting a company with a track record of success, a steady development pathway, and a disruptive solution that we believe could transform OA treatment for millions.
25
issued patents protecting their technology
Over
$25 million
raised
Strategic investments and partnerships from industry leaders


Nature's Blueprint, Improved
We knew the future of using A2M to treat OA lay in engineering a better version of A2M — one that was more effective, easier to produce, and available to patients worldwide.
Enter CYT-108—a lab-synthesized, genetically engineered “super A2M” designed for superior efficacy. CYT-108 has demonstrated 200% more efficacy than natural A2M, and since it’s produced in a lab, it can be manufactured at scale and distributed globally.

Image is a computer-generated demo. CYT-108 is currently under development.
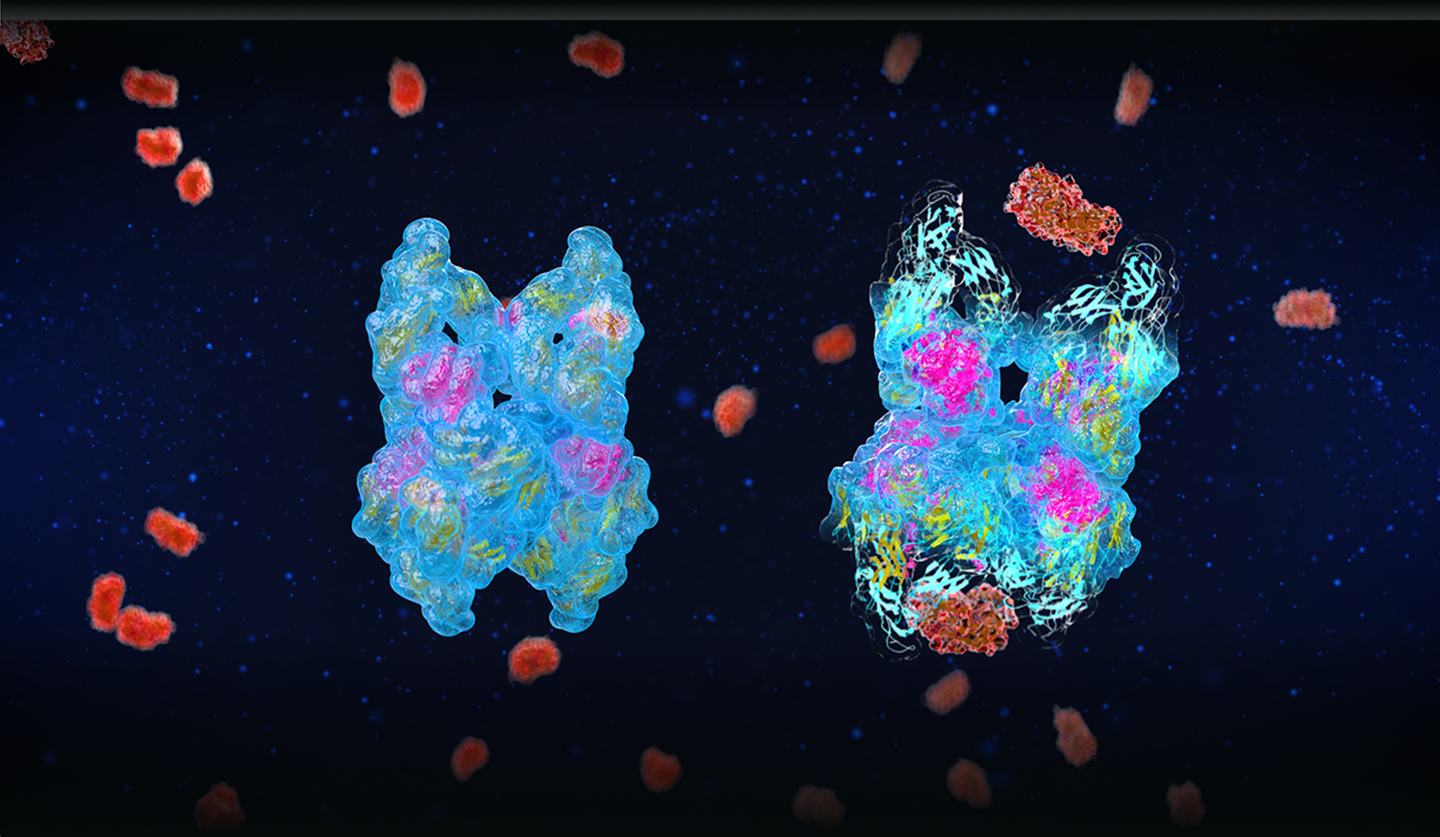
Comparison of the natural A2M protein (left) and Cytonics' engineered A2M variant, “CYT-108” (right), binding proteases (red). CYT-108 was engineered by creating genetic modifications to the “bait region” of the A2M protein to increase its ability to “recognize” specific proteases upregulated in arthritis joints and a wide range of more general proteases that also contribute to cartilage damage. It is this combination of hyper-specificity and broad-spectrum efficacy that differentiates CYT-108 from Big Pharma's failed attempts to develop a protease inhibitor treatment for OA. Simply put, we know that A2M works, and now we are aiming to “perfect" "Nature's blueprint”.
Imagine a Future Without Joint Replacements
One with fewer invasive surgeries like knee replacements. This is what we’re envisioning with CYT-108. Our strategy mirrors the evolution of insulin, which is a widely-used medicine based on something the body usually produces naturally. Before 1982, it was extracted from the pancreas – and then Eli Lilly engineered a synthetic version for mass production. This strategy of iterating on Nature's beautiful design is tried and true, and we believe that CYT-108 is the natural evolution of our powerful Alpha-2-Macroglobulin therapies.
We are mere months away from the most critical milestone in our conclusion of our Phase 1 clinical trial. This first-in-human study will provide us with valuable information about the safety and efficacy of CYT-108, and will be used to inform the design of our upcoming Phase 2 clinical trial. So far, CYT-108 appears well-tolerated when injected directly into patients' arthritic knees.
InvestWe are mere months away from the most critical milestone in our conclusion of our Phase 1 clinical trial. This first-in-human study will provide us with valuable information about the safety and efficacy of CYT-108, and will be used to inform the design of our upcoming Phase 2 clinical trial. So far, CYT-108 appears well-tolerated when injected directly into patients' arthritic knees.
Harnessing the Power of A2M for a Breakthrough in Osteoarthritis Treatment

Alpha-2-Macroglobulin ("A2M", blue/green) binding the destructive proteases responsible for cartilage degradation in arthritic joints. A2M is a naturally occurring protein found in the blood that plays a role in clotting. We discovered that we could leverage A2M's mechanism of action to treat osteoarthritis by introducing high concentrations of purified A2M from patient's own blood.
Image is a computer-generated demo. CYT-108 is currently under development.
At Cytonics, we didn’t invent the solution to OA — Nature did.
Alpha-2-Macroglobulin (A2M) is a large protein that plays a role in the blood clotting cascade. Naturally found in the blood, A2M functions as an anti-coagulant through a mechanism called protease inhibition. Proteases are catabolic enzymes that "chew" up other proteins, playing a crucial role in many normal, physiological processes (such as "protein turnover"). However, proteases can become dysregulated and contribute to disease... such as osteoarthritis.
In arthritic joints, proteases become upregulated and hyperactive as part of the natural aging process, or due to trauma. These hyperactive proteases chew through cartilage, destroying the protective cushioning within joints and producing cartilage protein fragments within the joint space. These fragments initiate an inflammatory cascade that ultimately results in more protease expression and increased inflammation. The only way to put a stop to this deleterious feedback loop is to stop the proteases in their tracks.
In arthritic joints, proteases become upregulated and hyperactive as part of the natural aging process, or due to trauma. These hyperactive proteases chew through cartilage, destroying the protective cushioning within joints and producing cartilage protein fragments within the joint space. These fragments initiate an inflammatory cascade that ultimately results in more protease expression and increased inflammation. The only way to put a stop to this deleterious feedback loop is to stop the proteases in their tracks.
The Protease Predator
That's where A2M comes in. Cytonics discovered A2M's ability to inhibit most classes of proteases responsible for osteoarthritis. However, the natural A2M levels in joints are far too low to lend any therapeutic benefit. A2M's broad-spectrum efficacy is a truly remarkable consequence of Nature's beauty. If only there was a way to harness this miraculous molecule to treat arthritic joints...

Alpha-2-Macroglobulin ("A2M", blue/green) binding the destructive proteases responsible for cartilage degradation in arthritic joints. A2M is a naturally occurring protein found in the blood that plays a role in clotting. We discovered that we could leverage A2M's mechanism of action to treat osteoarthritis by introducing high concentrations of purified A2M from patient's own blood.
Image is a computer-generated demo. CYT-108 is currently under development.
APIC: The First Proof of A2M’s Power
Our first-generation therapy, The Autologous Protease Inhibitor Concentrate (APIC Treamtent), leverages naturally occurring A2M to treat OA. How it works:



Extracts A2M from a patient’s blood
Purify A2M using proprietary filtration system
Inject selectively enriched A2M concentrate into arthritic joints

With this model, APIC has successfully treated over 10,000 patients, providing clinical and commercial proof that the A2M protein is an effective therapy for osteoarthritis.
Despite APIC’s success, it has limitations. It requires specialized equipment, training, and time. Plus, because it’s classified as a medical device rather than a drug, it isn’t covered by insurance. APIC was the first step towards an effective treatment because it proved the therapeutic power of A2M, but we knew that we could do even better.
Despite APIC’s success, it has limitations. It requires specialized equipment, training, and time. Plus, because it’s classified as a medical device rather than a drug, it isn’t covered by insurance. APIC was the first step towards an effective treatment because it proved the therapeutic power of A2M, but we knew that we could do even better.
Image is a computer-generated demo. CYT-108 is currently under development.
APIC™ Therapy
Our customized OA solution
- Licensed to 2 independent distributors for a $4M+ deal value with distributors, plus $400K annual royalties.
- Sold directly to physicians by a national medical device distributor since 2015.
Concentrates the body's natural A2M protease inhibitor


Direct joint injection into arthritic joints
APIC™ is Patient Tested, Physician Approved

A2M has been instrumental in treating my patients with cartilage injuries that are otherwise not amenable to cartilage restoration procedures... I have returned the majority of my patients who received an A2M injection to participation in sports successfully without the use of a scalpel.
- David E. Dominguez, MD, President/Owner: 3D Sports Medicine and Orthopedic Center

I have been using Cytonics' APIC kits to treat various joint pains (mostly in the knee). This is part of my regenerative medicine practice. I've seen remarkable results such that I have suggested that my wife and my son undergo treatments as well as patients.
- Laurence Rosenfield, MD, orthopedist, advocate of APIC treatment for patients and family

I had a single treatment of Cytonics' APIC therapy, APIC. Within 2 days the swelling and stiffness was gone and hasn't returned 6 months later. I was so impressed with these results that I have been evangelizing for APIC treatment to my doctors and friends ever since.
- Gabe, patient and APIC advocate

After almost eight months of therapy and various treatments, Richard Grossman, MD told me about Cytonics and the available APIC treatment. I received my first injection in April of 2018 and within weeks the large nodule in my Achilles had shrunk significantly. While I was feeling much better and able to start playing basketball and tennis again.
- Daryle Bobb, patient
*The above testimonials may not be representative of the opinions of other medical experts or the experiences of other customers and is not a guarantee of future performance or success.
InvestKey Achieved & Planned Milestones

2019-2023
Successfully completed all IND-enabling studies

Q2 2024
Launched Phase 1 clinical trial

Q3 2025
Full Phase 1 Clinical Study Report planned

EARLY 2026
Phase 2 clinical trial planned



2019-2023
Successfully completed all IND-enabling studies

ONGOING
Launched Phase 1 clinical trial

Q4 2025
Investigational New Drug (IND) application submission planned

*These forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially.
A Grassroots Revolution in Biotech
Biotech investing has typically belonged to Venture Capitalists. Cytonics is different: We are on a mission to Democratize Biotech Investing ripping the power out of Wall Street's tight grasp and giving it back to the people. We want that power to go to the people. We’re not looking for a handful of ultra-wealthy investors or Wall Street funds to take over; instead, we want to present the opportunity to invest in biotechnology research and development to everyday people.
Help us take on Big Pharma and Wall Street by investing in Cytonics today.
Help us take on Big Pharma and Wall Street by investing in Cytonics today.
Join The Movement
Oops! Something went wrong while submitting the form.
By clicking "Learn More", you consent to receive marketing text messages (e.g. promos, cart reminders) from StartEngine Crowdfunding, Inc. at the number provided, including messages sent by autodialer. Consent is not a condition of purchase. Msg & data rates may apply. Msg frequency varies. Unsubscribe at any time by replying STOP or clicking the unsubscribe link (where available). Privacy Policy & Terms
Why Invest in Cytonics?
Cytonics is developing what may be the one of the first and only effective therapies for osteoarthritis. If successful, we could succeed where Big Pharma has failed, and do so without selling out to Wall Street funds.
Treat the Disease
Cytonics is tackling OA at its molecular source with CYT-108, a targeted therapy designed to stop cartilage breakdown and give over 365M suffering patients their lives back.
First-In-Class
Cytonics is creating real solutions, not just temporary fixes. If successful, we will develop one of the first disease-modifying therapies for OA, addressing a $400B global market that is in desperate need of a solution.
Beat Big Pharma
Big Pharma has failed to develop a disease-modifying therapy for OA, leaving patients with only treatment for symptoms but not the disease itself. Our science-first approach may succeed where they have failed. If approved, CYT-108 may overcome the limitations of Big Pharma's failed approach
Put Patients Above Profits
Venture capital and Big Pharma control biotech funding, limiting innovation and prioritizing profit over patients. We refuse to play by their rules—our funding comes from everyday investors, not corporate backers.
A Grassroots Effort
6,000+ investors have already joined us in democratizing medical innovation. We have raised over $25M in total, with $15M from equity crowdfunding.
A Robust & Multilayered IP Fortress
We hold 25 international patents, securing our independence from Big Pharma’s monopolistic grip in key global markets (USA, EU, JP, AU, CA).
Brand Ambassadors
We are thrilled to be joined by Rener (of SharkTank fame!) and Eve Gracie (former WWE Diva), a professional martial arts power-couple that has experienced the magic of Cytonics' APIC(TM) therapy first hand. The Gracie's are one of Cytonics largest investors and most vocal advocates for our technology and R&D. Watch the video below to learn why they partnered with Cytonics and became shareholders.
*The above testimonials may not be representative of the opinions of other medical experts or the experiences of other customers and is not a guarantee of future performance or success.
Meet the Minds Behind Cytonics
Cytonics is led by a powerhouse team of executives and medical advisors in the biotechnology industry and regenerative medicine sector. With decades of combined expertise, strategic partnerships with industry leaders, and a proven track record of bringing medical innovations to market, we believe Cytonics is positioned for success in revolutionizing OA treatment.
Executive Board
Medical Advisory Board
Join Cytonics
Be Part of the Solution
We're on a mission to eradicate the pain and suffering of osteoarthritis. With $25M in funding to date, we invite you to join us on our journey– to pave the way for a brighter future in osteoarthritis treatment.
Join us in a grassroots effort to reclaim biotech innovation from Big Pharma and Wall Street.
InvestJoin us in a grassroots effort to reclaim biotech innovation from Big Pharma and Wall Street.
OFFERING STATEMENT REGARDING THIS OFFERING HAS BEEN FILED WITH THE SEC. THE SEC HAS QUALIFIED THAT OFFERING STATEMENT, WHICH ONLY MEANS THAT THE COMPANY MAY MAKE SALES OF THE SECURITIES DESCRIBED BY THE OFFERING STATEMENT. IT DOES NOT MEAN THAT THE SEC HAS APPROVED, PASSED UPON THE MERITS OR PASSED UPON THE ACCURACY OR COMPLETENESS OF THE INFORMATION IN THE OFFERING STATEMENT.
THE OFFERING CIRCULAR THAT IS PART OF THAT OFFERING STATEMENT CAN BE FOUND HERE.
CROWDCHECK VERIFIED REPORT CAN BE FOUND HERE.
THE OFFERING MATERIALS MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY’S MANAGEMENT. WHEN USED IN THE OFFERING MATERIALS, THE WORDS “ESTIMATE,” “PROJECT,” “BELIEVE,” “ANTICIPATE,” “INTEND,” “EXPECT” AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS, WHICH CONSTITUTE FORWARD LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT’S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY’S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE. THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO REVISE OR UPDATE THESE FORWARD-LOOKING STATEMENTS TO REFLECT EVENTS OR CIRCUMSTANCES AFTER SUCH DATE OR TO REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS.
THE OFFERING CIRCULAR THAT IS PART OF THAT OFFERING STATEMENT CAN BE FOUND HERE.
CROWDCHECK VERIFIED REPORT CAN BE FOUND HERE.
THE OFFERING MATERIALS MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY’S MANAGEMENT. WHEN USED IN THE OFFERING MATERIALS, THE WORDS “ESTIMATE,” “PROJECT,” “BELIEVE,” “ANTICIPATE,” “INTEND,” “EXPECT” AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS, WHICH CONSTITUTE FORWARD LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT’S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY’S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE. THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO REVISE OR UPDATE THESE FORWARD-LOOKING STATEMENTS TO REFLECT EVENTS OR CIRCUMSTANCES AFTER SUCH DATE OR TO REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS.
Exclusive Investor Perks


Venture Club
Venture Club Members
earn 10% bonus shares on top of this and all eligible investments for an entire year. Not a member? Sign up at checkout ($275/year).
Multiple investments in an offering cannot be combined to qualify for a larger campaign perk. Get rewarded for investing more into Cytonics.
The 10% StartEngine Venture Club Bonus.
Cytonics Corp will offer 10% additional bonus shares for all investments that are committed by investors that are eligible for the StartEngine Venture Club.
This means eligible StartEngine shareholders will receive a 10% bonus for any shares they purchase in this offering. For example, if you buy 100 shares of Common Stock at $3.00 / share, you will receive 110 shares of Common Stock, meaning you’ll own 110 shares for $300. Fractional shares will not be distributed and share bonuses will be determined by rounding down to the nearest whole share.
This 10% Bonus is only valid during the investor’s eligibility period. Investors eligible for this bonus will also have priority if they are on a waitlist to invest and the company surpasses its maximum funding goal. They will have the first opportunity to invest should room in the offering become available if prior investments are canceled or fail.
The 10% StartEngine Venture Club Bonus.
Cytonics Corp will offer 10% additional bonus shares for all investments that are committed by investors that are eligible for the StartEngine Venture Club.
This means eligible StartEngine shareholders will receive a 10% bonus for any shares they purchase in this offering. For example, if you buy 100 shares of Common Stock at $3.00 / share, you will receive 110 shares of Common Stock, meaning you’ll own 110 shares for $300. Fractional shares will not be distributed and share bonuses will be determined by rounding down to the nearest whole share.
This 10% Bonus is only valid during the investor’s eligibility period. Investors eligible for this bonus will also have priority if they are on a waitlist to invest and the company surpasses its maximum funding goal. They will have the first opportunity to invest should room in the offering become available if prior investments are canceled or fail.
Silver
invest
$10,000+
Invest $10,000+ and receive 10% bonus shares + 1 hour call with Joey Bose (President and CEO).
Gold
invest
$20,000
Invest $20,000+ and receive 15% bonus shares + all Silver Tier Company Perks, plus a conversation with Gaetano Scuderi, MD (Founder and board-certified orthopedic surgeon) regarding any musculoskeletal ailments you or a loved one are suffering from.
Diamond
invest
$50,000
Invest $50,000+ and receive 20% bonus shares + all Silver and Gold Tier Company Perks.
Platinum
invest
$100,000
Invest $100,000+ and receive 20% bonus shares + receive all Silver and Gold Tier Company Perks, plus free APIC treatment, including our FDA-approved therapy for osteoarthritis (note: we will cover the cost of the APIC kit plus the physician’s visit fee for administering the therapy, but not the initial consultation).
Loyalty Bonus | 5% Bonus Shares
Prior investors in the Company are eligible for 5% Bonus Shares as a loyalty bonus regardless of the amount of shares of Common Stock they purchase in this Offering.
Bonus Shares based on the amount of investment will be based on a single transaction and are not cumulative of multiple purchases. If an investor who is a member of the StartEngine Venture Club, is a previous investor, and/or also qualifies for Bonus Shares based on the amount they invest, then the Bonus Shares are stackable; however, the maximum amount of Bonus Shares that an investor can receive in this offering is 20%. To illustrate the issuance of Bonus Shares, if you are eligible for a 10% bonus from membership in the StartEngine Venture Club and buy 100 shares of Common Stock at $3.00 per share, you will receive 100 shares of Common Stock at that purchase price of $300 plus will be issued an additional 10 shares for no additional consideration. Fractional shares will not be distributed, and share bonuses will be determined by rounding down to the nearest whole share.
Prior investors in the Company are eligible for 5% Bonus Shares as a loyalty bonus regardless of the amount of shares of Common Stock they purchase in this Offering.
Bonus Shares based on the amount of investment will be based on a single transaction and are not cumulative of multiple purchases. If an investor who is a member of the StartEngine Venture Club, is a previous investor, and/or also qualifies for Bonus Shares based on the amount they invest, then the Bonus Shares are stackable; however, the maximum amount of Bonus Shares that an investor can receive in this offering is 20%. To illustrate the issuance of Bonus Shares, if you are eligible for a 10% bonus from membership in the StartEngine Venture Club and buy 100 shares of Common Stock at $3.00 per share, you will receive 100 shares of Common Stock at that purchase price of $300 plus will be issued an additional 10 shares for no additional consideration. Fractional shares will not be distributed, and share bonuses will be determined by rounding down to the nearest whole share.
Multiple investments in an offering cannot be combined to qualify for a larger campaign perk. Get rewarded for investing more into Cytonics.
The 10% StartEngine Venture Club Bonus.
Cytonics Corp will offer 10% additional bonus shares for all investments that are committed by investors that are eligible for the StartEngine Venture Club.
This means eligible StartEngine shareholders will receive a 10% bonus for any shares they purchase in this offering. For example, if you buy 100 shares of Common Stock at $3.00 / share, you will receive 110 shares of Common Stock, meaning you’ll own 110 shares for $300. Fractional shares will not be distributed and share bonuses will be determined by rounding down to the nearest whole share.
This 10% Bonus is only valid during the investor’s eligibility period. Investors eligible for this bonus will also have priority if they are on a waitlist to invest and the company surpasses its maximum funding goal. They will have the first opportunity to invest should room in the offering become available if prior investments are canceled or fail.
Loyalty Bonus | 5% Bonus Shares
Prior investors in the Company are eligible for 5% Bonus Shares as a loyalty bonus regardless of the amount of shares of Common Stock they purchase in this Offering.
Bonus Shares based on the amount of investment will be based on a single transaction and are not cumulative of multiple purchases. If an investor who is a member of the StartEngine Venture Club, is a previous investor, and/or also qualifies for Bonus Shares based on the amount they invest, then the Bonus Shares are stackable; however, the maximum amount of Bonus Shares that an investor can receive in this offering is 20%. To illustrate the issuance of Bonus Shares, if you are eligible for a 10% bonus from membership in the StartEngine Venture Club and buy 100 shares of Common Stock at $3.00 per share, you will receive 100 shares of Common Stock at that purchase price of $300 plus will be issued an additional 10 shares for no additional consideration. Fractional shares will not be distributed, and share bonuses will be determined by rounding down to the nearest whole share.
The 10% StartEngine Venture Club Bonus.
Cytonics Corp will offer 10% additional bonus shares for all investments that are committed by investors that are eligible for the StartEngine Venture Club.
This means eligible StartEngine shareholders will receive a 10% bonus for any shares they purchase in this offering. For example, if you buy 100 shares of Common Stock at $3.00 / share, you will receive 110 shares of Common Stock, meaning you’ll own 110 shares for $300. Fractional shares will not be distributed and share bonuses will be determined by rounding down to the nearest whole share.
This 10% Bonus is only valid during the investor’s eligibility period. Investors eligible for this bonus will also have priority if they are on a waitlist to invest and the company surpasses its maximum funding goal. They will have the first opportunity to invest should room in the offering become available if prior investments are canceled or fail.
Loyalty Bonus | 5% Bonus Shares
Prior investors in the Company are eligible for 5% Bonus Shares as a loyalty bonus regardless of the amount of shares of Common Stock they purchase in this Offering.
Bonus Shares based on the amount of investment will be based on a single transaction and are not cumulative of multiple purchases. If an investor who is a member of the StartEngine Venture Club, is a previous investor, and/or also qualifies for Bonus Shares based on the amount they invest, then the Bonus Shares are stackable; however, the maximum amount of Bonus Shares that an investor can receive in this offering is 20%. To illustrate the issuance of Bonus Shares, if you are eligible for a 10% bonus from membership in the StartEngine Venture Club and buy 100 shares of Common Stock at $3.00 per share, you will receive 100 shares of Common Stock at that purchase price of $300 plus will be issued an additional 10 shares for no additional consideration. Fractional shares will not be distributed, and share bonuses will be determined by rounding down to the nearest whole share.
Join the Discussion
Please check your email for the One Time Password. Didn't Receive an email? click Here
Your investment account has some outstanding AML/KYC issues that need your attention. Please help us resolve these by clicking Here and addressing the issues listed in your account.
Your investment account has some outstanding AML/KYC issues that need your attention. Please help us resolve these by clicking Here and addressing the issues listed in your account.
We'll email you once we are done reviewing your account. If it's been longer than 5 days since you addressed all issues, please email us at contact@startengine.com.
The community wants to hear your thoughts. Create an investment account HERE to join the discussion.
Your investment account has some outstanding AML/KYC issues that need your attention. Please help us resolve these by clicking HERE and addressing the issues listed in your account.
Learn Why Investors Have Invested $25M+ into Cytonics
Oops! Something went wrong while submitting the form.
By clicking "Learn More", you consent to receive marketing text messages (e.g. promos, cart reminders) from StartEngine Crowdfunding, Inc. at the number provided, including messages sent by autodialer. Consent is not a condition of purchase. Msg & data rates may apply. Msg frequency varies. Unsubscribe at any time by replying STOP or clicking the unsubscribe link (where available). Privacy Policy & Terms







































.svg)