Beating Pharma at Their Own Game
Osteoarthritis (OA) is an epidemic affecting 500M+ people, causing debilitating pain, complete loss of mobility, and with no real cure. Cytonics is righting Big Pharma’s repeated failures by developing the first truly disease-modifying therapy for OA.
10,000+ patients treated with first-generation therapy
$25M+ raised from 7,000+ investors, with $2M from pro athletes
Tracking toward a potential exit by 2028
Join this grassroots effort to reclaim biotech innovation from Big Pharma and Wall Street as an early-stage investor.
Osteoarthritis Therapeutics is a $560B+ 1 Market
There is no disability more common worldwide than arthritis, with osteoarthritis (OA) making up nearly half of those cases. That’s why its market for therapeutics is worth $560B+. With rates growing 132%2 worldwide between 1990 and 2020, and prevalence expected to rise another 60-100% by 2050, we need answers.

But No Drug Works
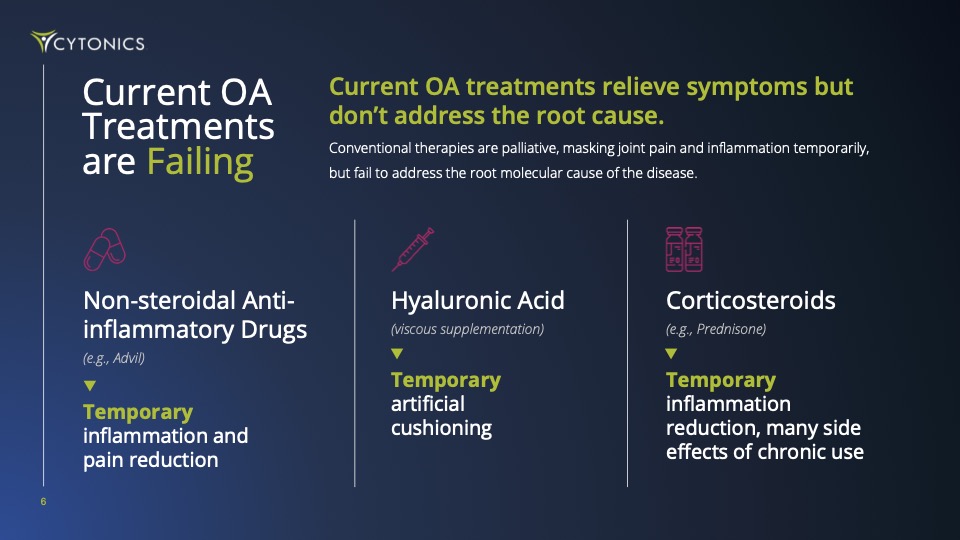
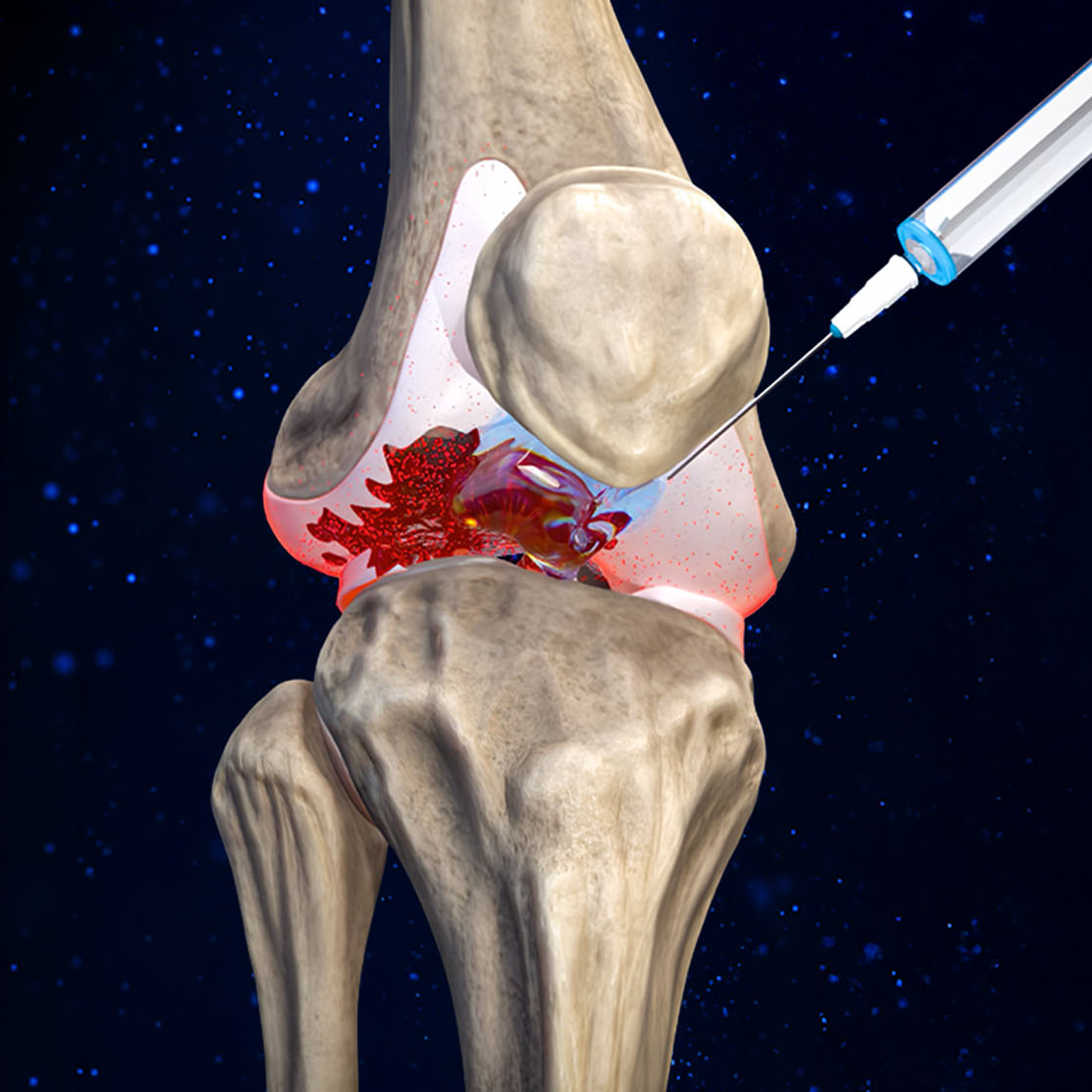
That means 500M+ global patients are getting short-term fixes like anti-inflammatories and corticosteroid shots. These may dull pain, but the disease still progresses toward costly, painful, and invasive joint replacements.
Big Pharma has spent $1B+ chasing a true “disease-modifying” therapy capable of halting OA in its tracks and repairing cartilage tissue, but their strategy has failed to appreciate the multi-faceted nature of the disease and they’ve consistently come up short.

Here’s Why Big Pharma is Failing
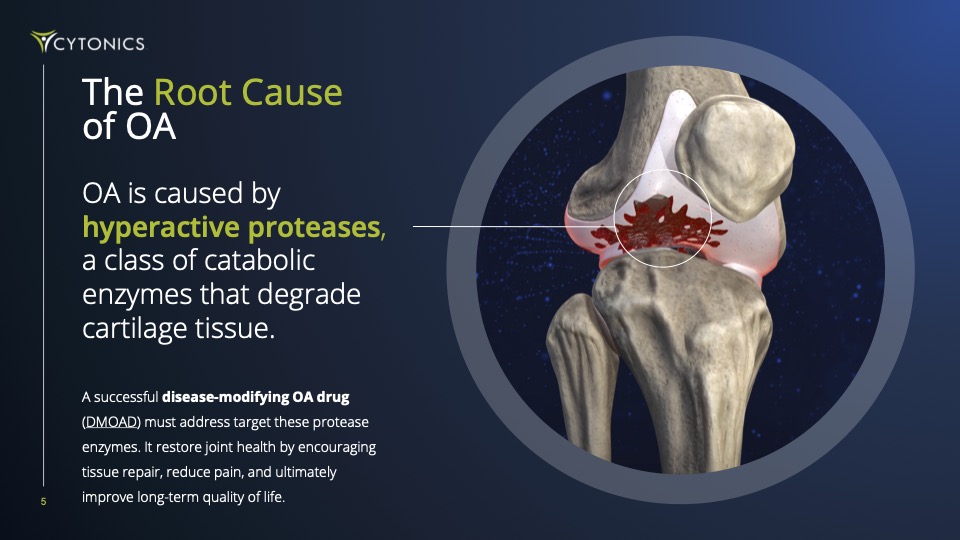
Every drug fails for the same reason: they go after just one target. But OA is complex, caused by a team of cartilage-destroying enzymes called “proteases”. If you don’t stop them all, the damage continues and the disease spirals out of control. Further compounding their shortsighted approach, they’ve attempted to deliver drugs orally, not directly into the joint.
CYT-108: The First Disease-Modifying Therapy for OA
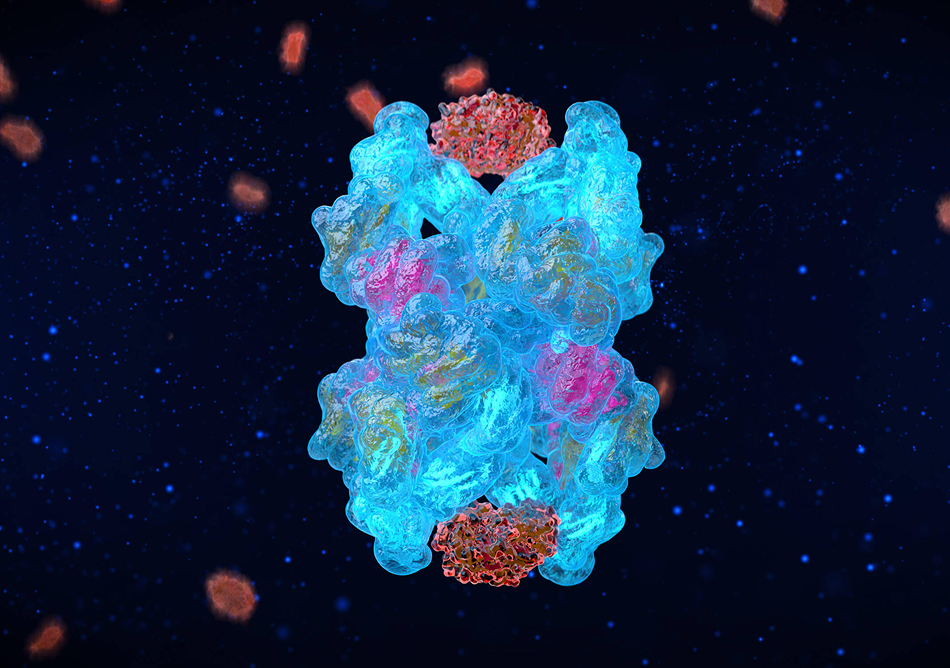
We made 31 precise genetic edits to the natural A2M gene sequence to give CYT-108 exceptional functionality as a protease inhibitor, creating this "Super A2M" variant that is 200% more effective than the natural A2M protein in halting cartilage degeneration and stimulating its regeneration.
1
2
3
Get the investor deck
A Grassroots Effort: For the People, By the People
We’re part of a grassroots movement to reclaim biotech innovation from Big Pharma and Wall Street. But our disruptions don’t stop there. For decades, early-stage biotech investing was reserved for VCs, hedge funds, and “institutional investors.” By inviting individuals like yourself to invest, we’re letting you share in the growth and commercialization of breakthrough science that tackles massive unmet needs like osteoarthritis.
10,000+ Patients Treated.
$7M Licensing Deal.
87% Cartilage Protection.
10,000+ patients already treated with first-gen therapy
Partnered with leading researchers like Stanford and Scripps


$7M licensing deals in place for our APIC therapy
Received $1.8M in National Institute of Health grants
CYT-108’s Phase 1 FDA human clinical trial completed; safety confirmed
25+ patents issued worldwide in key global markets (US, EU, CA, JP, AU)
Get the investor deck

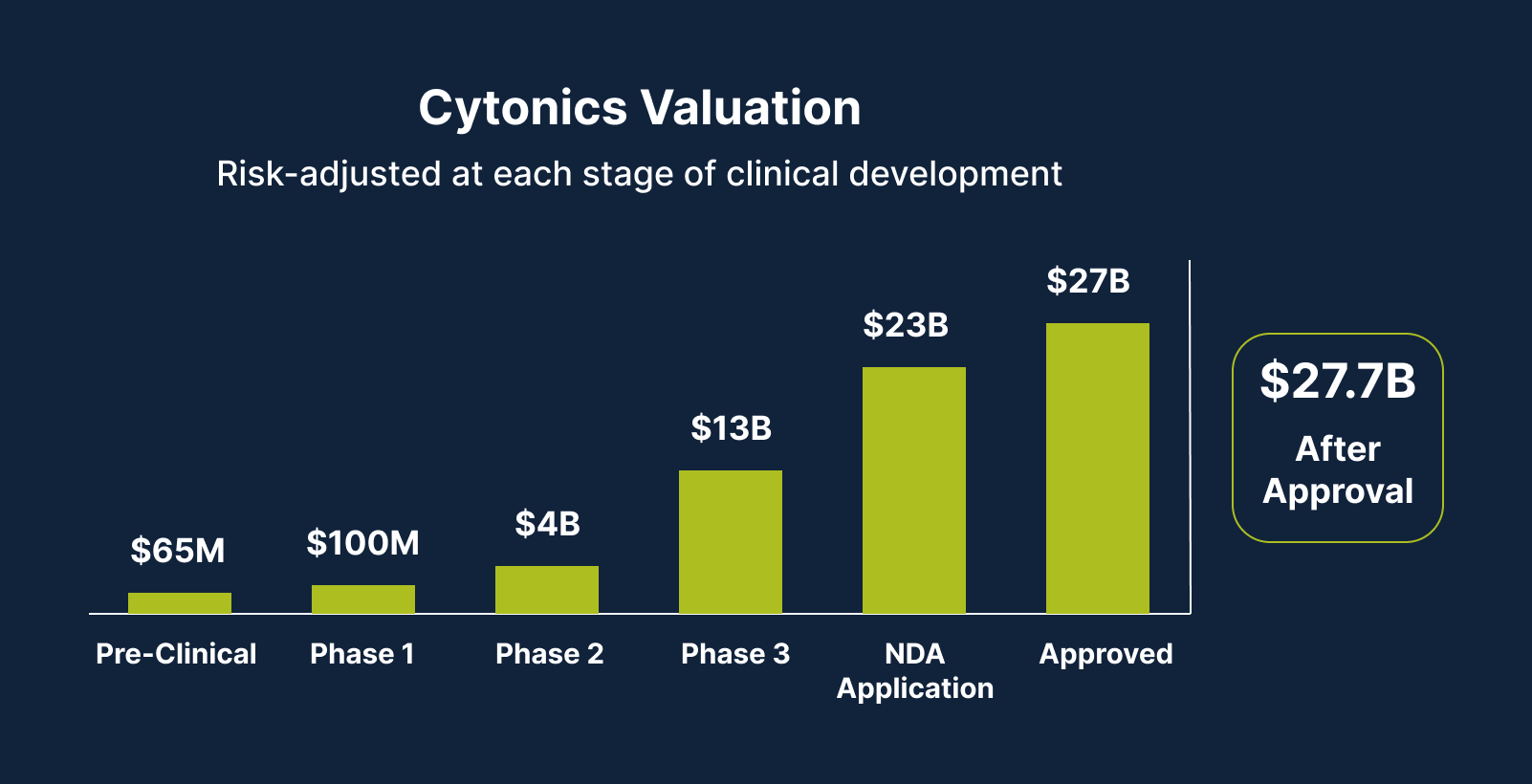
Our Path to Up to 270X Share Price Growth
As we currently sit at a Phase 1 valuation of $160M, we’re targeting a potential 270X exit upon our device’s approval. Here’s how we plan to get there:
2025
- Completed first-in-human Phase 1 clinical trial
- No drug-related adverse events were detected, confirming CYT-108’s safety
- Prepared next clinical trial (Phase 1b/2a) protocol
- Onboarded statistician, regulatory consultant (former FDA reviewer), and medical writers
- Initiated new Oncology program (focus on metastatic melanoma)
2026
- Publish Phase 1 Clinical Study Report
- Hold Type C meeting with the FDA to get feedback on Phase 1b/2a trial design (Q2)
- Launch Phase 1b/2a efficacy study (Q4)
2027–2028
- Complete Phase 1b/2a study (Q2)
- Hold meeting with FDA to discuss Phase 2b/3 clinical development program (Q3)
- Initiate Phase 3 pivotal trials (Q4)
- Position for IPO, acquisition, or major partnership to fund global commercialization
2029+
- Prepare regulatory submissions in US, EU, and select global markets
- Commercial launch of CYT-108 for osteoarthritis
- Continue to expand development pipeline into other disease areas (e.g., oncology)
- Pursue clinical development of additional A2M-based therapies
Strategically Partnered with Rener & Eve Gracie
A third-generation Brazilian Jiu-Jitsu master and former WWE champion, Rener and Eve Gracie’s seven-figure investment in Cytonics carries real weight. As two of the world’s most respected figures in athletics, injury prevention, and health advocacy, they bring credibility, visibility, and real-world expertise.

Health & Performance Advocacy:
Real-World Alignment:
Brand Expansion & Awareness:

“As a patient who has personally benefited from the Cytonics A2M therapy, I asked if I could be a partner to help spread the word. I have seen far too many times people get sidelined from their passions because their bodies don't respond and their bodies don't work and they exist in chronic pain. So if we can help reduce or eliminate that pain and delay the onset of chronic osteoarthritis, I'm all for it, sign me up."
Co-founder of Gracie University
Strategic partner/investor in Cytonics
Exclusive Investor Perks
Existing Investors Receive an Additional 10% in addition to the perks listed above.
Led by Seasoned Scientists, Physicians, and Industry Execs
Our leadership combines world-class scientific expertise, decades of clinical experience, and a proven track record in biotech commercialization. Our board members led groundbreaking orthopedic research, developed FDA-approved biologics at major pharma companies, and raised millions in capital. In full, we have a unique strategic advantage as we accelerate CYT-108 through trials, secure partnerships, and unlock exit opportunities.

- Proven innovator in orthopedic medicine, with 45+ peer-reviewed publications
- Served as faculty at Stanford and brings 30+ years of surgical experience
- Deep patient insight, leveraging decades of frontline experience treating joint degeneration

- 15+ years in biotechnology R&D with healthcare investment banking experience
- Led funding efforts to secure millions in non-VC investor backing
- Actively pursuing clinical trial execution, patent protection, and potential acquisition/licensing opportunities

- GMP and regulatory affairs veteran with deep experience in biologics and vaccine programs
- Trained microbiologist and immunologist
- Built and led quality systems and global inspection readiness efforts
- Supported INDs reviewed by FDA, EMA, MHRA, and NMPA
- Former BARDA and FDA Office of the Commissioner leader in medical countermeasures
- Leads GMP-compliant manufacturing of CYT-108 at Cytonics from day one

- Leads creative development and digital advertising at Cytonics
- Responsible for communicating Cytonics’ science, strategy, and long-term vision to investors and the public
- Specializes in translating complex biology into clear, disciplined narratives
- Extensive experience with early- and growth-stage companies in regulated, capital-intensive industries
- Oversees brand development, campaign strategy, and digital advertising supporting capital formation and investor education
- Focuses on precision-driven storytelling that maintains scientific and regulatory integrity
Frequently Asked Questions
Why invest in startups?
Regulation CF allows investors to invest in startups and early-growth companies. This is different from helping a company raise money on Kickstarter; with Regulation CF Offerings, you aren’t buying products or merchandise - you are buying a piece of a company and helping it grow.
How much can I invest?
Accredited investors can invest as much as they want. But if you are NOT an accredited investor, your investment limit depends on either your annual income or net worth, whichever is greater. If the number is less than $124,000, you can only invest 5% of it. If both are greater than $124,000 then your investment limit is 10%.
How do I calculate my net worth?
To calculate your net worth, just add up all of your assets and subtract all of your liabilities (excluding the value of the person’s primary residence). The resulting sum is your net worth.
What are the tax implications of an equity crowdfunding investment?
We cannot give tax advice, and we encourage you to talk with your accountant or tax advisor before making an investment.
Who can invest in a Regulation CF Offering?
Individuals over 18 years of age can invest.
What do I need to know about early-stage investing? Are these investments risky?
There will always be some risk involved when investing in a startup or small business. And the earlier you get in the more risk that is usually present. If a young company goes out of business, your ownership interest could lose all value. You may have limited voting power to direct the company due to dilution over time. You may also have to wait about five to seven years (if ever) for an exit via acquisition, IPO, etc. Because early-stage companies are still in the process of perfecting their products, services, and business model, nothing is guaranteed. That’s why startups should only be part of a more balanced, overall investment portfolio.
When will I get my investment back?
The Common Stock (the "Shares") of Cytonics (the "Company") are not publicly-traded. As a result, the shares cannot be easily traded or sold. As an investor in a private company, you typically look to receive a return on your investment under the following scenarios: The Company gets acquired by another company. The Company goes public (makes an initial public offering). In those instances, you receive your pro-rata share of the distributions that occur, in the case of acquisition, or you can sell your shares on an exchange. These are both considered long-term exits, taking approximately 5-10 years (and often longer) to see the possibility for an exit. It can sometimes take years to build companies. Sometimes there will not be any return, as a result of business failure.
Can I sell my shares?
Shares sold via Regulation Crowdfunding offerings have a one-year lockup period before those shares can be sold under certain conditions.
Exceptions to limitations on selling shares during the one-year lockup period:
In the event of death, divorce, or similar circumstance, shares can be transferred to:
• The company that issued the securities;
• An accredited investor;
• A family member (child, stepchild, grandchild, parent, stepparent, grandparent, spouse or equivalent, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law, or sister-in-law, including adoptive relationships).
What happens if a company does not reach their funding target?
If a company does not reach their minimum funding target, all funds will be returned to the investors after the close of the offering.
How can I learn more about a company's offering?
All available disclosure information can be found on the offering pages for our Regulation Crowdfunding offering.
What if I change my mind about investing?
You can cancel your investment at any time, for any reason, until 48 hours prior to a closing occurring. If you’ve already funded your investment and your funds are in escrow, your funds will be promptly refunded to you upon cancellation. To submit a request to cancel your investment please email: info@dealmakersecurities.com
How do I keep up with how the company is doing?
At a minimum, the company will be filing with the SEC and posting on its website an annual report, along with certified financial statements. Those should be available 120 days after the fiscal year end. If the company meets a reporting exception, or eventually has to file more reported information to the SEC, the reporting described above may end. If these reports end, you may not continually have current financial information about the company.
What relationship does the company have with DealMaker Securities?
Once an offering ends, the company may continue its relationship with DealMaker Securities for additional offerings in the future. DealMaker Securities’ affiliates may also provide ongoing services to the company. There is no guarantee any services will continue after the offering ends.